Battery:
A battery is an electrochemical device that stores electrical energy in chemical form and converts it into electrical energy when connected to a load through a chemical reaction between its electrodes and electrolyte.
When you connect a battery to a device, a chemical reaction (called a redox reaction) occurs inside, causing electrons to flow from one terminal to another through an external circuit, which powers your device.
How a Battery Works
Every battery has three main components:
- Anode (-): The negative electrode that releases electrons.
- Cathode (+): The positive electrode that receives electrons.
- Electrolyte: A chemical medium (liquid or gel) that allows ions to move between the electrodes while forcing electrons to travel through the external circuit.
Types of Batteries
Batteries are generally classified into two main categories based on whether they can be recharged.
1. Primary Batteries (Non-Rechargeable)
These are “single-use” batteries. Once the chemical reactants inside are exhausted, they cannot be recharged and must be discarded.
- Alkaline Batteries: The most common household type (AA, AAA). Used in TV remotes, clocks, and flashlights.
- Zinc-Carbon: An older, cheaper alternative to alkaline, used in low-drain devices.
- Lithium (Primary): These offer high energy density and long shelf life. Used in smoke detectors and digital cameras.
- Silver Oxide: Small “button cells” used in watches and hearing aids.
2. Secondary Batteries (Rechargeable)
These can be recharged by applying an external electrical current, which reverses the internal chemical reaction.
- Lithium-Ion (Li-ion): The most popular type today. Lightweight with high energy density. Used in smartphones, laptops, and Electric Vehicles (EVs).
- Lead-Acid: One of the oldest types. They are heavy but can provide high surge currents. Used in car starters and UPS systems.
- Nickel-Metal Hydride (NiMH): Common in rechargeable AA/AAA packs. Used in cordless phones and some hybrid cars.
- Nickel-Cadmium (NiCd): Durable and works well in extreme temperatures, though largely replaced by NiMH due to toxic cadmium.
Comparison Table
| Feature | Primary Battery | Secondary Battery |
| Rechargeable? | No | Yes |
| Chemical Reaction | Irreversible | Reversible |
| Initial Cost | Low | High |
| Life Cycle | Single use | Hundreds to thousands of uses |
| Typical Use22 | Remotes, toys, watches23 | Phones, cars, laptops |
Alkaline Batteries:
An alkaline battery is a type of primary (non-rechargeable) battery that derives its energy from the chemical reaction between zinc metal and manganese dioxide.
It gets its name because it uses an alkaline electrolyte (typically potassium hydroxide) instead of the acidic electrolytes used in older zinc-carbon batteries.

How an Alkaline Battery Works
Alkaline batteries work through a “redox” (reduction-oxidation) reaction. When you connect the battery to a device, electrons begin to flow to provide power.
- At the Anode (Negative Terminal): The anode is made of zinc powder. The zinc reacts with the hydroxide ions in the electrolyte, releasing electrons. This process is called oxidation.
- Reaction: Zn(s) + 2OH–(aq) → ZnO(s) + H2O(l) + 2e–
- At the Cathode (Positive Terminal): The cathode is made of manganese dioxide (MnO2). It “pulls” the electrons coming from the external circuit and uses them to transform the manganese dioxide into manganese oxide-hydroxide. This is called reduction.
- Reaction: 2MnO2(s) + H2O(l) + 2e– → Mn2O3(s) + 2OH–(aq)
- The Electrolyte: The potassium hydroxide (KOH) acts as a bridge. It stays chemically constant throughout the process but allows ions to move inside the battery to balance the electrical charge as electrons move outside.
Result: This movement of electrons through your device (from the flat negative end to the nubby positive end) is what provides the 1.5V of electricity.
Advantages
- High Energy Density: They store 3–5 times more energy than standard zinc-carbon batteries, meaning they last much longer.
- Long Shelf Life: They have a very low self-discharge rate, retaining up to 90% of their power even after being stored for 5–10 years.
- Safety: They are generally stable, non-toxic (most modern ones are mercury-free), and safe to carry on planes.
- Temperature Resistance: They perform well in a wide range of temperatures (from roughly -20°C to 55°C).
- Cost-Effective: They are inexpensive and widely available in standard sizes like AA, AAA, C, D, and 9V.
Disadvantages
- Non-Rechargeable: Once the chemicals inside are used up, the battery is dead. Recharging a standard alkaline battery can cause it to leak or even explode.
- Risk of Leakage: As they discharge, they produce hydrogen gas which can build up pressure. If left in a device for too long after they die, the seals can fail, leaking a corrosive “white crust” (potassium carbonate) that can ruin electronics.
- High Internal Resistance: While great for remotes and clocks, they can struggle with “high-drain” devices like digital cameras, where they might “die” quickly because they can’t pump out enough current fast enough.
- Bulkier than Lithium: Compared to lithium disposables, alkaline batteries are heavier and larger for the same amount of power.
Zinc-carbon battery:
A zinc-carbon battery (also known as a Dry Leclanche cell) is the oldest and simplest type of commercial dry cell battery. It is a primary (non-rechargeable) battery that is often labeled as “General Purpose” or “Heavy Duty” in stores.
While they have been largely replaced by alkaline batteries for high-performance needs, they remain very common because they are extremely cheap to manufacture.

How a Zinc-Carbon Battery Works
The battery converts chemical energy into electrical energy through a redox reaction involving zinc and manganese.
- The Anode (Negative): The outer shell of the battery is actually made of zinc metal. It serves as both the container and the negative terminal. During use, the zinc dissolves into the electrolyte, releasing electrons.
- The Cathode (Positive): This is a carbon (graphite) rod in the center. It is surrounded by a paste of manganese dioxide (MnO2) and carbon powder. The carbon rod itself doesn’t react; it simply collects the electrons.
- The Electrolyte: A moist paste of ammonium chloride (NH4Cl) or zinc chloride (ZnCl2).
The Chemical Process
When the circuit is closed, the following happens:
- Oxidation at Anode: Zn(s) → Zn2+ (aq) + 2e–
- Reduction at Cathode: 2MnO2(s) + 2NH4+(aq) + 2e– → Mn2O3(s) + 2NH3(aq) + H2O(l)
- The electrons flow through your device to get from the zinc shell to the carbon rod.
Advantages
- Very Low Cost: They are the cheapest batteries on the market.
- Widely Available: You can find them almost anywhere, often sold in bulk.
- Ideal for Low-Drain Devices: They are perfect for things that use very little power over a long time, such as wall clocks, TV remotes, and basic calculators.
- Reliable for Intermittent Use: They perform well in devices that are used only occasionally and then left sit.
Disadvantages
- Low Energy Density: They hold about 4 to 5 times less energy than an alkaline battery of the same size.
- High Leakage Risk: Because the zinc shell is consumed during the reaction, the “walls” of the battery eventually get thin and can develop holes. This allows the acidic electrolyte to leak out and corrode your electronics.
- Short Shelf Life: They lose their charge much faster than alkaline or lithium batteries, usually lasting only 1.5 to 3 years in storage.
- Poor High-Drain Performance: If you put them in a digital camera or a high-powered toy, they will “die” in minutes because they cannot provide a high current consistently.
- Voltage Drop: The voltage drops steadily as the battery is used, which cause devices might like flashlights to get dimmer and dimmer rather than staying bright.
Silver Oxide battery:
A silver oxide battery (IEC code: S) is a primary (non-rechargeable) battery known for its high energy density and extremely stable voltage. You will most commonly see these as “button cells” in devices that require precision, like high-end watches or medical instruments.
While they look identical to alkaline button cells (like the LR44), silver oxide batteries (like the SR44) use silver as the cathode, making them more expensive but significantly higher-performing.

How it Works
The silver oxide battery operates through a chemical reaction between zinc and silver oxide in an alkaline environment.
- The Anode (Negative): Made of Zinc powder.
- The Cathode (Positive): Made of Silver Oxide (Ag2O).
- The Electrolyte: Usually Potassium Hydroxide (KOH) or Sodium Hydroxide (NaOH).
The Chemistry
- At the Anode: Zinc reacts with the electrolyte to release electrons.
- Zn + 2OH– → ZnO + H2O + 2e–
- At the Cathode: The silver oxide receives those electrons and is reduced to pure metallic silver.
- Ag2O + H2O + 2e– → 2Ag + 2OH–
The key takeaway: Unlike alkaline batteries where the voltage drops steadily as you use them, silver oxide batteries maintain a very “flat” discharge curve—meaning they stay at a nearly constant 1.55V until they are almost completely dead.
Advantages
- Stable Voltage: This is their biggest “pro.” Because the voltage doesn’t drop until the very end, they are perfect for watches, digital calipers, and light meters that need exact power to remain accurate.
- High Energy Density: They store about twice as much energy as an alkaline battery of the same size.
- Long Shelf Life: They can sit in a drawer for 5 to 10 years and still retain about 90% of their original charge.
- Leakage Resistance: They are much less likely to leak compared to alkaline button cells, making them safer for expensive electronics.
- Operating Range: They perform well in cold temperatures (down to -20°C).
Disadvantages
- High Cost: Silver is an expensive precious metal. A silver oxide button cell can cost 2–3 times more than an alkaline equivalent.
- Non-Rechargeable: Like other primary batteries, they cannot be recharged.
- Small Sizes Only: Due to the cost of silver, you won’t find “AA” silver oxide batteries. They are almost exclusively limited to small button or coin sizes.
- Environmental Concern: Older models contained mercury to prevent corrosion (though most modern ones are now labeled “Mercury Free”)
Lithium-Ion (Li-ion) battery:
Lithium-Ion (Li-ion) batteries are at the heart of modern electronics—and they’re fascinating from an electrical engineering point of view. A Lithium-Ion (Li-ion) battery is a rechargeable battery in which lithium ions move between the anode and cathode through an electrolyte during charging and discharging, providing high energy density, low weight, and long cycle life.

Construction (Main Components)
- Anode (Negative Electrode)
- Usually made of graphite
- Stores lithium ions during charging
- Cathode (Positive Electrode)
- Made of lithium metal oxides such as:
- LiCoO₂ (Lithium Cobalt Oxide)
- LiFePO₄ (Lithium Iron Phosphate)
- LiMn₂O₄ (Lithium Manganese Oxide)
- Made of lithium metal oxides such as:
- Electrolyte
- Lithium salt (e.g., LiPF₆) dissolved in organic solvent
- Allows movement of lithium ions
- Separator
- Thin porous membrane
- Prevents short circuit while allowing ion flow
- Current Collectors
- Copper (anode) and aluminum (cathode)
Working Principle
During Discharging
- Lithium ions move from anode → cathode through electrolyte
- Electrons flow through external circuit
- Electrical energy is supplied to the load
During Charging
- External power source forces lithium ions from cathode → anode
- Energy is stored chemically in the battery.
Key Characteristics
| Parameter | Typical Value |
| Nominal Voltage | 3.6 – 3.7 V per cell |
| Energy Density | 150 – 250 Wh/kg |
| Efficiency | 90 – 95% |
| Cycle Life | 500 – 2000 cycles |
| Self-discharge | Low (~2–3% per month) |
Advantages
- High energy density
- Lightweight and compact
- Low self-discharge
- No memory effect
- Fast charging capability
Disadvantages
- Expensive compared to lead-acid
- Sensitive to overcharge and overheating
- Requires protection circuit
- Capacity degrades over time
Applications
- Smartphones and laptops
- Electric vehicles (EVs)
- Power banks
- Solar energy storage
- Medical devices
- Drones and robotics
Lead-Acid Battery
Definition
A Lead-Acid battery is a rechargeable electrochemical battery that uses lead dioxide (PbO₂) as the positive plate, sponge lead (Pb) as the negative plate, and sulfuric acid (H₂SO₄) as the electrolyte.
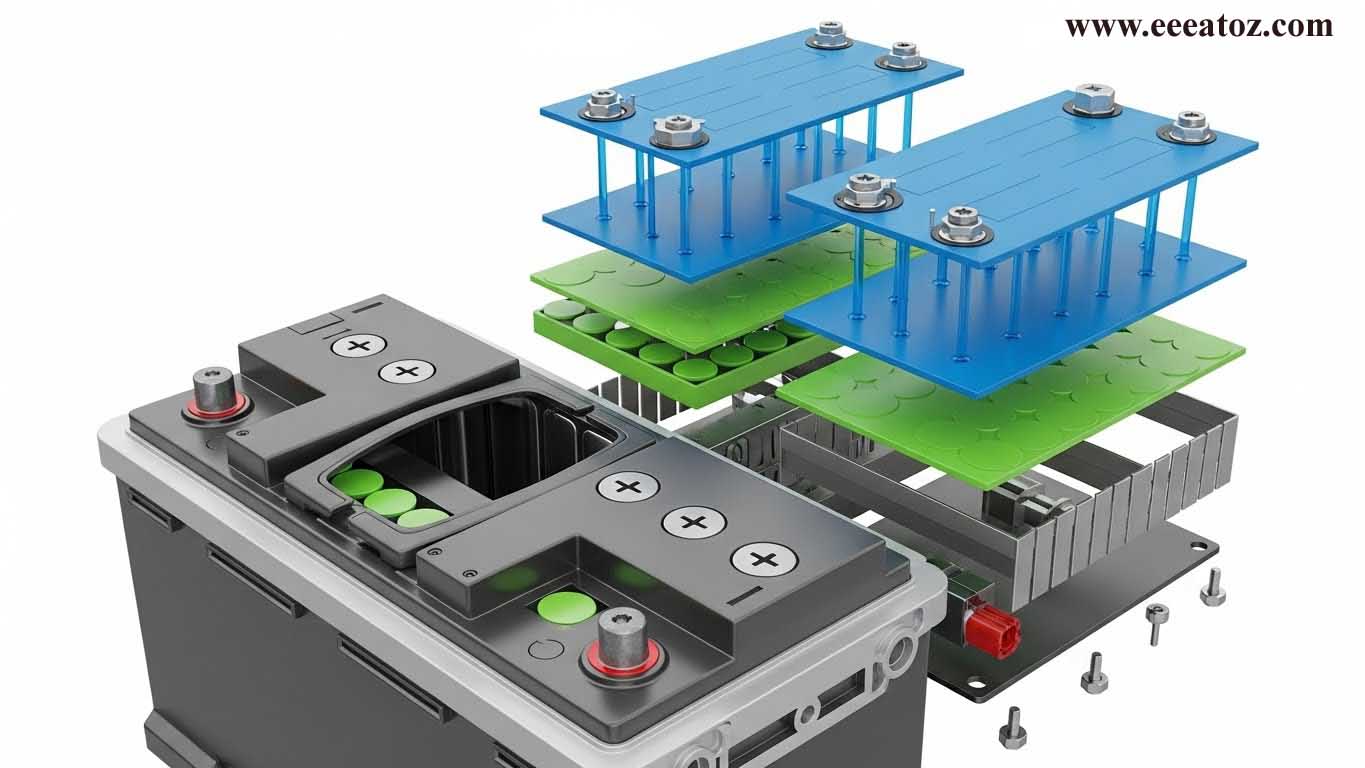
Construction
A typical lead-acid battery consists of:
- Positive Plate: Lead dioxide (PbO₂)
- Negative Plate: Spongy lead (Pb)
- Electrolyte: Dilute sulfuric acid (H₂SO₄)
- Separator: Porous insulating material (prevents short-circuit)
- Container: Hard rubber or plastic
- Terminals: Positive (+) and Negative (–)
Each cell produces approximately 2 V
A 12 V battery equal to 6 cells in series
Working Principle
(a) During Discharging
When the battery supplies current to a load:
- PbO₂ and Pb react with sulfuric acid
- Both plates convert into lead sulfate (PbSO₄)
- Water is formed, reducing electrolyte concentration
Chemical Reactions:
- At Positive Plate:
PbO₂ + H₂SO₄ + 2H⁺ + 2e⁻ → PbSO₄ + 2H₂O - At Negative Plate:
Pb + SO₄²⁻ → PbSO₄ + 2e⁻
(b) During Charging
When external DC supply is applied:
- Lead sulfate converts back into PbO₂ and Pb
- Sulfuric acid concentration increases
- Battery regains stored energy
Nominal Values
| Parameter | Value |
| Cell Voltage | 2 V |
| Fully Charged Cell | ≈ 2.2 V |
| Electrolyte SG (charged) | 1.26 – 1.28 |
| Electrolyte SG (discharged) | ≈ 1.12 |
Advantages
- Low cost
- Simple construction
- High surge current capability
- Reliable and robust
- Easily recyclable
Disadvantages
- Heavy and bulky
- Low energy density
- Limited life cycle
- Requires maintenance (in flooded type)
- Slow charging
Applications
- Automobile starting batteries
- UPS and inverters
- Emergency lighting
- Power stations and substations
- Solar power systems (backup)
Nickel-Metal Hydride (NiMH) Battery:
Definition
A NiMH battery is a rechargeable battery that uses nickel oxyhydroxide as the positive electrode and a metal hydride as the negative electrode.

Construction
- Positive Electrode: Nickel oxyhydroxide (NiOOH)
- Negative Electrode: Metal hydride alloy
- Electrolyte: Potassium hydroxide (KOH)
Working Principle of Nickel–Metal Hydride (NiMH) Battery
A Nickel–Metal Hydride (NiMH) battery operates through reversible electrochemical reactions involving a nickel-based positive electrode and a hydrogen-storage metal alloy negative electrode.
During Discharging
When the battery supplies power:
- The metal hydride electrode releases stored hydrogen.
- The nickel oxyhydroxide electrode undergoes a reduction reaction.
- Electrons travel through the external circuit from the negative to the positive terminal, producing usable electrical energy.
During Charging
When an external voltage is applied:
- Electrical current is driven in the reverse direction.
- Hydrogen is forced back into the metal alloy electrode.
- The chemical materials return to their original state, preparing the battery for the next discharge cycle.
Advantages
- Higher capacity than Ni-Cd
- Environment-friendly (no cadmium)
- Moderate energy density
- Rechargeable
Disadvantages
- Higher self-discharge than Li-ion
- Lower efficiency
- Generates heat during fast charging
- Shorter life than Li-ion
Applications
- Rechargeable AA/AAA cells
- Hybrid vehicles
- Cordless phones
- Cameras
Quick Comparison Table
| Feature | Li-ion | Lead-Acid | NiMH |
| Energy Density | Very High | Low | Medium |
| Weight | Light | Heavy | Medium |
| Cost | High | Low | Medium |
| Maintenance | No | Yes | No |
| Memory Effect | No | No | Very Low |
| Applications | EVs, Mobile | Cars, UPS | Rechargeable cells |
FAQ:
A. Short Questions
What is a battery?
A battery is an electrochemical device that converts chemical energy into electrical energy.What is the basic working principle of a battery?
A battery works on the principle of oxidation and reduction reactions.Name the two main electrodes of a battery.
Anode and Cathode.What is the function of electrolyte in a battery?
It allows the flow of ions between electrodes.What happens at the anode during discharge?
Oxidation occurs and electrons are released.What happens at the cathode during discharge?
Reduction occurs and electrons are accepted.Define primary battery.
A battery that cannot be recharged once discharged.Define secondary battery.
A battery that can be recharged and reused.Give two examples of primary batteries.
Dry cell, Alkaline battery.Give two examples of secondary batteries.
Lead-acid battery, Lithium-ion battery.What is battery capacity?
The amount of charge a battery can deliver, usually in ampere-hours (Ah).What is EMF of a battery?
The open-circuit voltage of the battery.
B. Very Short Questions
Unit of battery capacity → Ah
Ion flow occurs through → Electrolyte
Electron flow occurs through → External circuit
Rechargeable battery example → Li-ion
Non-rechargeable battery example → Dry cell
C. Multiple Choice Questions (MCQs)
1. A battery converts
A) Electrical energy to mechanical energy
B) Chemical energy to electrical energy
C) Mechanical energy to electrical energy
D) Heat energy to electrical energy
Answer: B
2. Which part of a battery releases electrons during discharge?
A) Cathode
B) Electrolyte
C) Anode
D) Separator
Answer: C
3. Which of the following is a primary battery?
A) Lead-acid
B) Ni-Cd
C) Lithium-ion
D) Dry cell
Answer: D
4. Which battery is commonly used in automobiles?
A) Lithium-ion
B) Alkaline
C) Lead-acid
D) Zinc-carbon
Answer: C
5. The electrolyte in a lead-acid battery is
A) Sodium chloride
B) Potassium hydroxide
C) Sulphuric acid
D) Distilled water
Answer: C
6. Which battery has high energy density?
A) Lead-acid
B) NiMH
C) Lithium-ion
D) Zinc-carbon
Answer: C
7. During charging of a battery
A) Chemical energy is converted to electrical energy
B) Electrical energy is converted to chemical energy
C) Heat energy is produced
D) No reaction occurs
Answer: B
8. The unit of EMF of a battery is
A) Ampere
B) Ohm
C) Watt
D) Volt
Answer: D
9. Which of the following is a secondary battery?
A) Alkaline
B) Dry cell
C) Mercury cell
D) Lead-acid
Answer: D
10. Which battery is widely used in mobile phones?
A) Lead-acid
B) Nickel-iron
C) Lithium-ion
D) Zinc-air
Answer: C
D. One-Line Conceptual MCQs (Quick Revision)
Oxidation occurs at → Anode
Reduction occurs at → Cathode
Rechargeability depends on → Reversibility of chemical reaction
Battery used in UPS → Lead-acid
Environment-friendly battery → NiMH / Li-ion